Frontiers in Correlated Matter:
Physicists
meet to discuss the intellectual challenges of
complex matter.
Piers Coleman and Leo Kadanoff
Piers Coleman and Leo Kadanoff
Main
Hard Matter
Soft and biological matter
At the meeting, there was a huge surge of interest in soft matter and biological systems. Condensed matter physics is generally divided in "hard" and "soft" condensed matter physics. "Hard" matter refers
to matter governed by atomic forces and quantum mechanics . Hard matter is generally not biological, and involves crystals, glasses, metals, semiconductors and oxides. "Soft" matter refers to the myriad varieties of matter that form on the macroscopic scale, often from biological, or organic molecules. Here the physics, though collective,
does not involve quantum mechanics, and the materials of interest are literally "softer".
 |
 |
 |
 |
Crumpled and Granular Matter
Towards
a science of Matter Singularities : Thomas Witten
Packings, Jammings, Correlations and Entropies in Condensed Matter and Candy : Paul Chaikin
Structure and
function of Biological matterPackings, Jammings, Correlations and Entropies in Condensed Matter and Candy : Paul Chaikin
The statics and dynamics of biological molecules
Electrodynamics
in soft matter
and biophysics: Phil Pincus
Mechanics and Statistical Mechanics of Living Cells :David Weitz
Matter with Memory:
Glasses and Gloopy Matter.Mechanics and Statistical Mechanics of Living Cells :David Weitz
Disciplined
Disorder and Disordered
Disciplines :Peter Wolynes
Why don't glasses flow? Philip Bouchaud
Why don't glasses flow? Philip Bouchaud
Crumpled and Granular Matter
One broad class of resarch work looked at how materials could fall into jammed configurations in which they would move very slowly, remember their past, and maintain interesting structures over long periods of time.
Much of the work was related to understanding the functioning of a groups of atoms or molecules by taking into account the shapes and forms they took. People looked at new kinds of materials which have a much richer family of shapes and stable behaviors than our usual materials. A bucket of water has shape given to it by the bucket. That is a familiar behavior. However, a bucket of sand may have your footprint or mine or anyone's. These materials with memory may have very interesting and novel properties.
|
|
 |
 |
Thomas Witten, University of Chicago pointed out the memory in a crumpled piece of paper (see figure). This paper can have sharp corners and folds which remain even after one tries to straighten out the paper. The corners themselves represent an energy focusing, and they give an example of how small objects may form from large. This problem is presented in a much larger context as parts of the study of the development of beautiful structures in the motion of fluids and elastic systems. A sheet of plastic, like a plastic bag can, when ripped, produce a riffled structure with riffles of all sizes included. Fluids can be made to come to a point form very thin necks. The time is ripe for a completion of our understanding of the kinds of structures which might be produced in these dynamics. These are fundamental investigations in mathematics and physics, with excellent prospects for practical applications. Witten speculated that there may be a whole new science of matter singularities connected with these observations. |
|
|
|
||
| The topic of memory was taken up by Jerry Gollub of Haverford College, in his discussion of research on flow in granular materials, like sand or sugar loose packed rocks or snow. Granular matter exists in two states: one is like a disordered gas, the other has the regular crystal lattice of a solid. | ||
 |
When sheared, perhaps trapped between two plates which maintain a fixed separation but move in opposite directions, random granular matter can crystallize, thereby dramatically changing its flow properties. In some cases both ordered and disordered states coexist, with the disordered state remaining around for a very long period, but finally being replaced by an ordered configuration. The transition from ordered to disordered states is not only affected by the obvious properties of the material, its density , state of shear, etc.--but also by less obvious properties build into the details of the placement of grains within the materials. |
|
These detailed relationships record the effect of the past history of the sample. Some of this "internal record-keeping" is understood, some remains to be understood. Additional puzzling issues include how and whether the distribution of grain-sizes affect the behavior, the observed transition from smooth to jerky motion, and the possible relation of this to fracture of materials. An open question important to people in the field is whether these materials can best be understood as a bunch of particles or as a kind of fluid in which the particle-behavior is hidden. |
||
|
|
 |
 |
In a slightly different vein, Paul Chaikin of New York University gave a brilliant description of how the packing of candy into a jar. This simple experiment has important stories to tell about how things may arrange themselves in space. He started by pointing out that, for an ordinary liquid, as the temperature goes down, the molecules will tend to fall into a configuration in which they will have the highest possible density. Sometimes that density is achieved by an ordered state, at other times a disordered state is the most tightly packed. In still other experimental runs the system gets stuck and does not have enough time to achieve its best configuration. The disordered configuration of a low-temperaure fluid is a glass. To construct a "model fluid", i.e. a simple and accessible analog of a real fluid, an undergraduate student at Princeton packed M&M s into a jar. The packing reached an unexpectedly high density. This in turn offered the possibility that some molecular shapes--close to the M&M-shape--might produce a material which remained a glassy fluid down to very low temperatures. Possibilities of new, ultraslow, fluids dance (or ooze) into view. |
The structure and function of Biological matter
Another class of work focused upon the forms and groupings of molecules in biological systems, and how those structures affect dynamical properties and biological functioning. Several of the participants discussed how ideas of condensed matter are starting to shape problems of biological complexity.
"Live Fluids": Ray Goldstein
Ray Goldstein from the University of Arizona described how fluids acquire new structure, and develop new flow patters in response to the forces derived from the flagella of bacteria.(see figure) Understanding such "live fluids" , he suggested - will require an innovative fusion of ideas from biology and fluid dynamics. 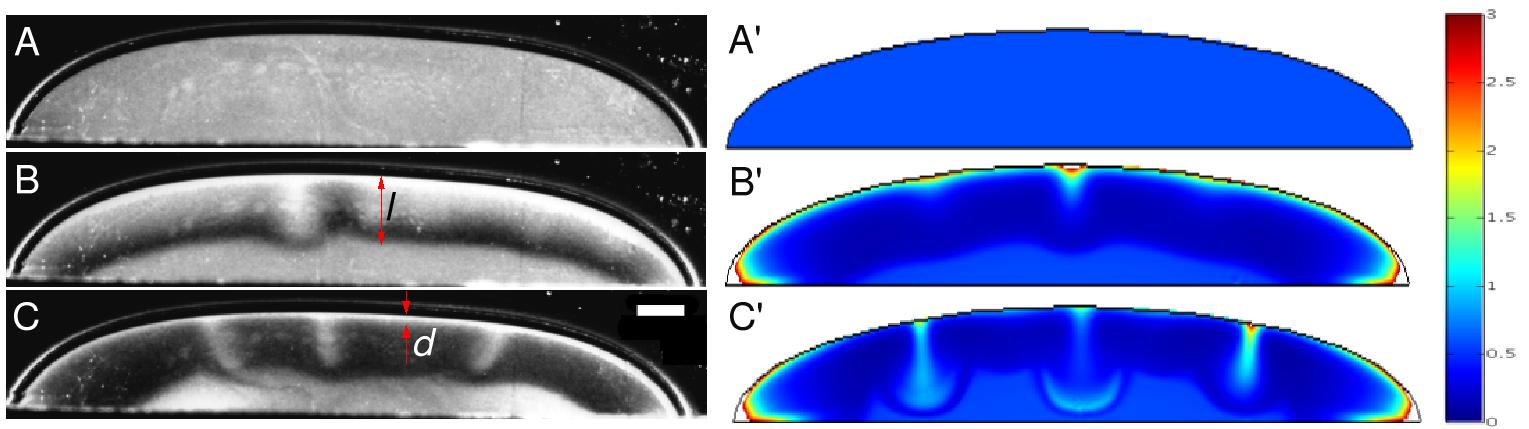 Formation of "pillars" in live fluid. |
 |
|
| He and his coworkers constructed a drop containing bacteria and sufficient food. However soon after the start of the experiment, the bacteria would begin to deplete the oxygen in the environment. They would then come together in pillars. Each pillar would swim up to the top of the fluid. The top would then become overloaded with the heavier than water bacteria. Their weight would then overturn the fluid. The overturn produced an overall flow pattern which then enables the bacteria to bunch up at the edge of the drop. In this way, they found a relatively favorable position for getting to the oxygen outside the drop. In this late stage, the bacteria would keep up a small-scale flow which looks superficially like turbulence. This experiment raises questions about how the bacteria communicate. Less immediately one might ask about interaction between bacteria and their environment and how they make use of their environment and of their communication possibilities. This is an interesting set of questions in which the tools developed by condensed matter's fluid mechanics community might be put to good use. | ||
Synthesis:
From Molecules to
Complexity: George Whitesides
 |
George Whitesides of Harvard University observed life and cells are just one of many complex systems that populate the world, including weather and economic systems; large networks such as the nervous system, computers, information systems and energy distribution grids. Our ability to understand and control the complex behavior of these systems he argued, is a central problem in science and engineering. Whitesides expressed optimism that biology is slowly developing the kinds of reproducible experiments that bring it into the realm of physics. In Physics and Chemistry we are accustomed to sets of rules and laws that govern our understanding - Newton laws, Arrhenius' laws, the application of statistical mechanics - but what, he asked, are the rules for biology? The answer, Whiteside conjectures, will lie in in discovering the principles that regulate and describe complex networks. |
|
Whitesides proposed that that many of the old problems of physics and chemistry were running a little thin so that it was time to find something new. His list of new topics included life and its origin, and also sentience intelligence and complexity, with psychology, sociology and economics being subjects which might become more ripe for investigation by physicists in the not-too-distant future. He pointed out that biology was developing the competence for reproducible and reliable experiments which would bring it into a state appropriate for further investigation by the techniques of physics. Examples of important opportunities include the application of dynamical system theory to the networks that control cell function and to such more extended systems as blood clotting. An additional application is to the motility of flexible cells, where the major question is whether motion follows cell-shape or shape responds to cellular motion. The shapes and structures of important biomolecules are determined by an interplay between the physical and the chemical properties of the molecules which make them up.
The statics and dynamics of biological molecules
Electrodynamics in soft matter and biophysics: Phil Pincus
| Phil Pincus
from UC Santa Barbara asked what is the the physical
mechanism connected with the functioning of the long-chain biological
molecules,
actin and DNA? Both molecules feel important forces from their
own
electrical charges, and from the chargers drawn to them in
solution. Pincus
pointed out that we have recently achieved a pretty good understanding
of the static properties of the complex structures which form around
the molecules as
they sit in solution. The next decades show promise of giving us
an
understanding of the crucial dynamical properties of these surrounding
layers.
Some of this dynamics will come from the universal properties of the
electrical
charges in these structures. Other pieces, involving molecular
recognition,
will come from more detailed "chemical" properties produced by the
details of the molecules and their environment. The use of
physics and chemistry
together to explain biological phenomena is likely to be a hallmark of
important
near future investigations in this area. |
 |
|
Mechanics
and Statistical
Mechanics of
Living Cells :David Weitz
 |
Dave Weitz of
Harvard University, extended the discussion of biology
from liquid properties to those of elastic networks. Part of this
discussion was an illustration of the use of in vitro simulations of
biological systems. Cells use networks of actin--a molecule in the form
of a long
chain-- to provide themselves rigidity and strength. These qualities
arise when
the actions are connected and welding into a kind of network. To
construct an artificial structure like that in the cell, he placed
together three
proteins, actin, a second protein which cross-linked the actin, and a
third which
helps cut the actin into the right lengths. He then studied the
stretching properties of this artificial system and compared it to the
stretching
of cells. He found a detailed correspondence between the two,
enabling him to argue that it was just this network which produced the
observed
behavior of cells. He then supplemented this work with a study of
twisting in
a real cell. He added a dye which could fluoresce to the actin of
a
cell, which is arranged into a network of tubes. Using this dye
he can observe
the actual motion as it occurs in a living cell. This pair of
studies shows
the beginning of a set of work which we might hope will teach us about
cells by constructing their parts and comparing the behavior of aptly
constructed parts with that of the real thing. |
|
Matter with Memory: Glasses.
 |
Disciplined Disorder and Disordered Disciplines:Peter Wolynes |
|
Memory effects were taken up by Peter Wolynes of UC San Diego and J-P Bouchard from Saclay, France, in their surveys of recent advances in our understanding of glasses. Glasses have the capacity of memory as as concomitant of their being able to be responsive or "adaptive". Glasses have this property. The detailed local arrangement of atoms provides a sort of trap in which the material gets stuck or "jammed" (that is the technical word used in this subject). As a result a glassy material, like common window glass, is a kind of fluid, which however shows great resistance to flow. Glasses "remember" when they were formed. Their configuration slowly changes or "ages". |
||
Why
don't
glasses flow?
|
 |
|
| Both speakers argues that the behavior of our familiar glasses was produced by partially realized phase transitions. There are two such transitions. The higher temperature transition dropped the fluid into a glassy state in which the system permitted jumping back and forth between some sorts of configurations but greatly inhibited jumps to other configurations. As the system aged, it slowly found more favorable configurations. However, it could not directly return to the previous, less favorable, ones. If the temperature were lowered still further, the system would undergo another phase transition and fall into a disordered low temperature state. However this lower temperature transition is almost always preempted by a drop into a solid configuration. This conceptual framework was used to describe how structures form within the glasses, how these structures get jammed, how glasses age, and how the material retains memory of its past. | ||
 |
|
|
Michael Cates, from the University of Edinburgh, talked about how memory and landscapes become very important at the interface between biology and soft matter, playing a vital role in our understanding of how matter self-assembles. In glassy systems, he said, this is question of learning how to steer soft matter through an energy landscape. A huge class of soft matter is "gloopy" - matter which flows like a liquid, but which remember's its shape on short time-scales, so that it can be cut with a knife, and it flows in sudden bursts, as in the classic example of ketchup "glooping" out of the bottle. |
  |
|
| The frivolous
question
"why
does gloop gloop?" actually encodes a huge number of unsolved questions
in
physics, such as what is different about the interactions of molecules
inside gloopy
matter? How do these interactions control when flow starts and
stops.
Examples of gloopy matter include polymers - molecular spaghetti -
colloids- fluids containing molecular billiard balls - and even
sand. These
materials might be thought of as a generalized class of
"glasses". |
||
 |
Cates described
his group's work on colloids colloids of lecithin inside a bile salt,
in which a
suddent change in the concentration of bile salt causes the disk-like
colloids
of lecithin to become unstable, starting to evolve into spherical
colloids. Gloopy matter, forms because this evolution becomes arrested
in a state containing a network of curved bilayers - or
vesicules. Cates
emphasized that by looking at such mixtures in parameter regimes that
are far outside
those seen in real biological matter, they have been able to learn far
more
about the mechanism by which the gloopy matter develops. In
particular,
Cate's group has been able to show that like a glass, the gloopy matter
is
actually metastable, and they've been able to simulate the formation of
these
arrested states.
|
|
Cates ended by remarking that the problem of understanding how non-equilibrium matter moves through a complex energy landscape is a central issue that needs to be addressed in the context of both nano and biological physics. There's lots of new physics out there, he says, but we can't apply it until we understand it first. |
||